 |
 |
|
NUTRISTEM HUMAN EMBROYNIC STEM CELL MEDIA |
MOUSE EMBRYONIC STEM CELL PRODUCTS |
FBS- ES QUALIFIED |
MESENCHYMAL STEM CELL QUALIFIED MEDIA |
The intricate relationship between genetics and embryonic development has both fundamental and practical implications for human health and disease. Stem cell research is probably one of the most exciting areas in the field of biology today due to the fact that it is advancing scientific knowledge exponentially in leaps and bounds, and raises as many questions as rapidly as it generates potential new discoveries. This promising area of scientific investigation provides a mechanism for creating animal-free component models with enormous therapeutic potential in Human Embryonic Stem Cell (hESC) research, and has also presented unforeseen and often startling insights into the role genes play in normal growth and development. Creating animal models of important human genetic diseases like cystic fibrosis (CF) or hemophilia is not only a prerequisite for developing and testing gene therapy, but embryonic stem cell technology also provides a testing ground to correct and repair genetic defects in utero.
Nutrients and other niche requirements for expanding the populations of therapeutic cells on a commercial scale are quite different from those needed for the production of cells in culture. An Animal Component-Free medium is a very important characteristic in the development of stem cells and regenerative medicine, as tissues are designed for implantation directly into humans(1). Human embryonic and adult stem cells each have different characteristics regarding their potential use for cell-based regenerative Although serum supplementation has been, and probably will be, a crucial planning step that plays a vital and essential role in the success of your final medium in the near future, our Serum-Free (SF) and Animal Component-Free (ACF) medium has been successfully tested for Human Embryonic Stem Cell Culture. Each and every hESC batch is not only tested for its ability to maintain pluripotency and its differentiation capability, but also its normal morphology and karyotype in a serum-free environment. Until now, there were simply no real viable alternatives for a human embryonic stem cell culture without animal-derived components of any kind. Fortunately or unfortunately, serum or serum-like replacements were and still are necessary for the growth and proliferation of cells in culture. Although they have been characterized as an ill-defined "black box" mixture of all types of proteins, structural, carrier and functional proteins including growth factors, hormones, minerals, trace elements and even inhibitory substances, their inherently indefinable and wide variation of components with accompanied downtime for pre-screening and testing, have been all but eliminated with our fully defined, serum-free and animal componentfree medium. NutriStem™ hESC XF therapies. The most obvious difference in the way embryonic and adult stem cells diverge is in the numbers and types of differentiated cell types they can become. In theory, embryonic stem cells, on the one hand, are pluripotent cells that have the ability to differentiate into all cell types of the body. Adult stem cells, on the other hand, are generally limited in terms of differentiation to the cell type tissue of origin. However, some recent evidence does suggest adult stem cell plasticity may exist, thus increasing the number of cell types an adult stem cell can become(2).
The utilization of Human Embryonic Stem Cells with its enormous therapeutic potential requires the development of serum-free and animal componentfree media in order to close the gap between research models and clinical therapeutic procedures. This ever-increasing demand for media, free of any and all animal-derived components, is one of Biological Industries' top priorities.
|
- Genetic Engineering and Biotechnology News (GEN, Jan 15, 2009 pp 32-34). “Culture Media Underlies Productivity Gains,” by Angelo DePalma,.
- The National Institutes of Health, U.S. Department of Health and Human Services, 2006.
|
Although serum supplementation has been, and probably will be, a crucial planning step that plays a vital and essential role in the success of your final medium in the near future, our Serum-Free (SF) and Animal Component-Free (ACF) medium has been successfully tested for Human Embryonic Stem Cell Culture. Each and every hESC batch is not only tested for its ability to maintain pluripotency and its differentiation capability, but also its normal morphology and karyotype in a serum-free environment.
Until now, there were simply no real viable alternatives for a human embryonic stem cell culture without animal-derived components of any kind. Fortunately or unfortunately, serum or serum-like replacements were and still are necessary for the growth and proliferation of cells in culture. Although they have been characterized as an ill-defined "black box" mixture of all types of proteins, structural, carrier and functional proteins including growth factors, hormones, minerals, trace elements and even inhibitory substances, their inherently indefinable and wide variation of components with accompanied downtime for pre-screening and testing, have been all but eliminated with our fully defined, serum-free and animal componentfree medium. |
NutriStem™ hESC XF |
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. NIS |
|
| NutriStem™ hESC XF Xeno-Free medium for hESCs With HSA Optimized for FF culture, May be used with feeder-dependent culture Superior performance on Matrigel® |
05-100-1A
05-100-1B |
500ml
100ml |
-20ºC
-20ºC |
| AF NutriStem™ hESC XF Xeno-Free medium for hESCs Without HSA Optimized for feeder-dependent culture |
05-102-1A
05-102-1B |
500ml
100ml |
-20ºC
-20ºC |
|
| |
Defined, xeno-free serum-free media, designed to support the growth of human embryonic stem cells (hESCs)
Traditional human Embryonic Stem Cells (hESC) culture methods require the use of mouse or human fibroblast feeder layers, or feeder-conditioned medium. These culture methods are labor-intensive, hard to scale up, and it is difficult to maintain hES cells undifferentiated due to undefined conditions. NutriStem™ hESC XF media were developed with a leading group in stem cell research, to enable the maintenance and expansion of hESCs with feeder cells or on feeder-independent culture. NutriStem™ hESC XF support the culture of undifferentiated hESC in serum-free conditions without any animal components on mouse feeder cells (MEF), Matrigel or human foreskin fibroblasts (HFF). The media contain recombinant human basic fibroblast growth factor (rh bFGF) and recombinant human transforming growth factor (rh TGF). The media have been successfully tested and proven to maintain the pluripotential nature of hESCs.
For long-term growth of hESCs without feeder cells, the use of NutriStem™ hESC XF (with HSA) is recommended.
|
Features |
| Some predominant characteristics of our NutriStem™ hESC XF SF media include: |
- A complete ready-to-use formulation (no additions are required). Contains Alanyl glutamine.
- Xeno-free: all components are defined and are of non-animal origin
- Enables expansion of hESCs on feeder-free culture conditions (Matrigel®) on human-feeder layer (foreskin fibroblasts) or on Mouse feeder cells (MEFs) High
- attachment - does not require adaptation period for feeder free culture Enables
- superior expansion of hESCs
- Supports long-term growth of hESCs (over 25 passages)
- Maintains differentiation capability of hESCs
- Maintains robust pluripotency
- Maintains normal phenotype (colony morphology) and genotype (karyotype testing) of hESCs
- Low proteins, low FGF levels
- Provides gene expression profiles comparable to classical media
- Provides gene expression profiles comparable to classical media
- Intended for use in a 5% CO2 atmosphere (ordinary conditions)
- Consistent media performance
- Performance tested
- Sterile-filtered (0.1μ)
- Mycoplsma tested
- Endotoxin tested
|
Precaution and Disclaimer |
- For in vitro diagnostic and research use only.
- Do not use if a visible precipitate is observed in the medium.
- Do not use NutriStem™ hESC XF media beyond the expiration date indicated on the product label.
|
Storage and Stability |
NutriStem™ hESC XF should be stored at -20ºC. Upon thawing, the media may be stored at 2-8ºC for 2 weeks. Dispense into aliquots to avoid repeated freezing and thawing.
Protect the media from light.
|
Quality Control |
NutriStem™ hESC XF performance is tested for optimal maintenance and expansion of undifferentiated hESCs. Additional standard evalutions are pH, osmolality, endotoxins and sterility tests.
|
Figure 1a: |
hESCs (H9.2) growing on Matrigel® (passage 5) express pluripotency markers as tested by Q-PCR. The results are expressed as percentage from GAPDH expression.
|
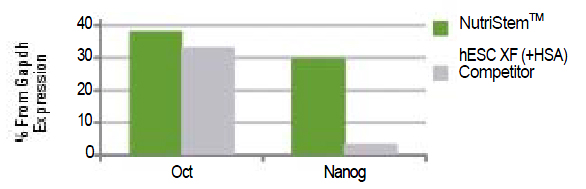 |
| |
Figure 1b: |
hESCs (H9.2) growing on Human Foreskin Fibroblasts (HFF) (passage 5) express pluripotency markers as tested by Q-PCR. The results are expressed as percentage from GAPDH expression.
|
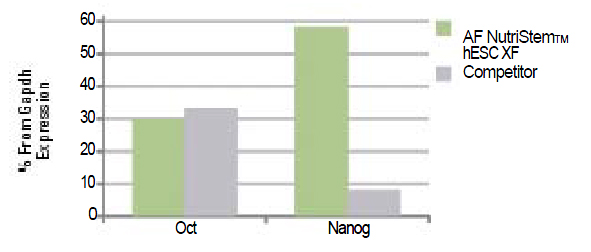 |
| |
Figure 2: |
Evaluation of NutriStem™ hESC XF cultured human embryonic stem cells (H9.2 cells) using Matrigel as a matrix. Growth promotion for hESCs cultured in NutriStem™ hESC XF versus leading competitor. Cell counts are reported for days 2, 4 and 7.
|
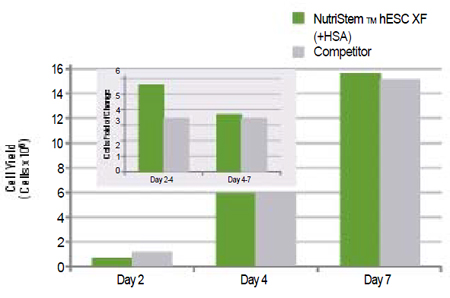 |
| |
Figure 3: |
hESCs (H9.2) growing on Human Foreskin Fibroblasts (HFF) (passage 3) express pluripotency markers as tested by Q-PCR. The results are expressed as percentage from GAPDH expression.
The control is a basic medium and supplements routinely used in a leading stem cell laboratory.
|
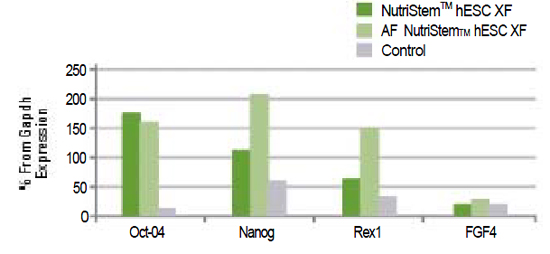 |
| |
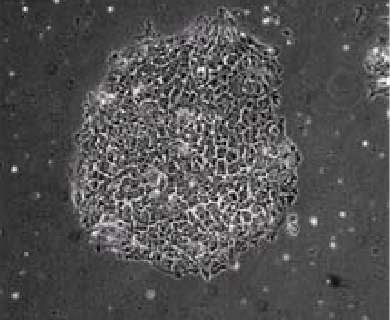
Figure 4: |
| NutriStem™ hESC XF - Matrigel® (>7 Passages). |
 |
| |
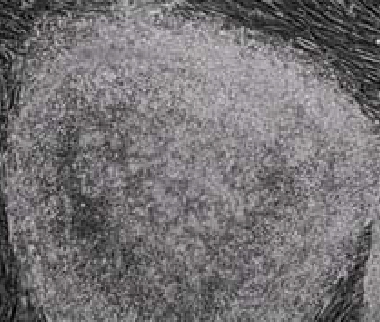
Figure 5: |
| NutriStem™ hESC XF - HFF (>7 Passages). |
 |
| |
Human Serum Albumin (HSA) |
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. |
|
| Bio-Pure Human Serum Albumin (HSA Solution, 10%), Optimized for Human Embryonic Stem Cells (hESC) |
05-720-1B
05-720-1E |
100ml
50ml |
-20ºC
-20ºC |
|
| |
HSA is a medium supplement that is a highly soluble osmolytic protein with a high molecular weight. It was specifically developed to support and maintain cell development, growth, health and productivity in most cell culture media and especially cell membrane stability. The primary function of HSA is not only its unique demonstrative capability of binding anionic, cationic and neutral molecules, but it also has the proclivity of sequestering and stabilizing a wide array of ions and other small molecules.
HSA complies with the specifications of the manufacturer and the requirements stipulated by FDA approved tests.
All individual donations of the plasma and the corresponding plasma pool, has been tested for Hepatitis B Surface Antigen (HBsAg), Anti (Human Immunodeficiency Virus) HIV-I and II and anti-HCV and found to be negative.
|
Figure 6: |
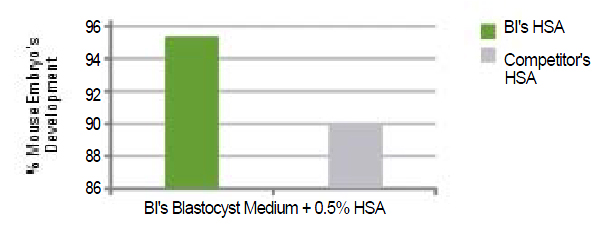 |
| |
Mouse Embryonic Stem Cells Basal Medium |
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. |
|
| Mouse Embryonic Stem Cells (ESC) Basal Medium With L-Alanyl L-Glutamine |
01-171-1A
01-171-1B |
500ml
100ml |
2-8ºC
2-8ºC |
|
| |
Basal medium designed for the growth of mouse embryonic stem (ES) cells
Mouse embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass of the blastocyst. Undifferentiated ES cells can be maintained in-vitro for extended periods without loss of their capacity to differentiate to all cell lineages when reimplanted back into a blastocyst. ES cells may differentiate in-vitro to a variety of cell types including neuronal, muscle, endothelial and hematopoietic progenitors. General culture conditions are well established and usually require ES cells to be grown on an inactive feeder cell layer or on gelatin-coated plates with Leukemia Inhibitory Factor (LIF) in the culture medium.
Mouse ES Basal Medium has been optimized to grow and maintain undifferentiated mouse embryonic stem cells. The medium may be used with the addition of Foetal Bovine Serum (FBS) or with any serum replacement designed for mouse ES cells.
The medium contains L-glutamine in a stable form.
|
Storage |
Mouse ES Basal Medium should be kept at 2-8ºC. Protect the medium from light.
|
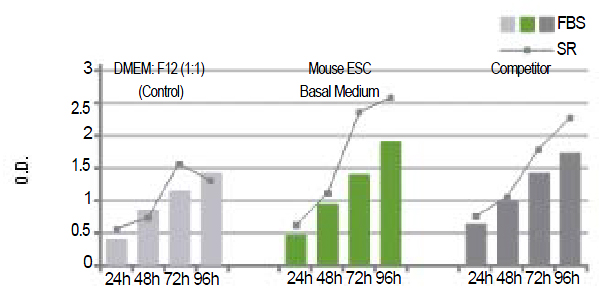
Figure 7: |
| Growth rate of mouse ESC (ES-D3) using Biological Industries' Mouse ESC Basal Medium vs. a competitor's medium. mESC were cultivated in a 96 well plates and observed over a period of 5 days. Proliferation rate was measured using a colorimetric method (XTT based Cell Proliferation Kit , Cat. No. 20-300-1000). Absorbance was read after 4 hours of incubation (wavelength of 450nm and reference of 690nm), proceeding addition of the XTT reagent. |
 |
| |
Gelatin Solution, 0.1% |
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. |
|
| Gelatin Solution (0.1%) |
01-944-1A
01-944-1B |
500ml
100ml |
2-8ºC
2-8ºC |
|
| |
Qualified for Mouse Embryonic Stem (ES) Cells
Gelatin solution (0.1%) is intended for coating cell culture flasks or plates used for the growth of mouse ES cells without feeder layer and with the addition of LIF to the culture medium.
|
Foetal Bovine Serum-Qualified for Human Embryonic Stem (ES) Cells |
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. |
|
| Certified Foetal Bovine Serum (FBS) Qualified for Human Embryonic Stem Cells |
04-002-1A
04-002-1B |
500ml
100ml |
-20ºC
-20ºC |
| Certified Foetal Bovine Serum (FBS) Qualified for Human Embryonic Stem Cells Heat Inactivated |
04-222-1A
04-222-1B |
500ml
100ml |
-20ºC
-20ºC |
|
| |
Recommended Applications |
For proliferation and differentiation of pluripotent embryonic stem cells. Based on high growth promotion parameters and lack of toxicity in highly sensitive human embryonic cell lines, this product is also recommended for sensitive mouse embryonic stem cells, human adult stem and primary cells.
In order to maintain highly viable stem cells in culture, high concentrations of growth factors, hormones and other growth stimulating additives are needed. To provide growth factors in constant concentrations, the FBS must be tested Foetal Bovine Serum, tested on human embryonic stem cells, is specifically tested for the ability to sustain undifferentiated cellular morphology of embryonic stem cells. Only suitable batches are selected and kept for stem research clients. Screening FBS batches for Biological Industries is performed at the Technion Institute of Technology in the Human Embryonic Stem Cells Laboratory of Prof. Joseph Itskovitz-Eldor M.D., D.Sc.,Faculty of Medicine-Stem Cells Research Center in Israel. The group at the Technion has extensive experience in the derivation and maintenance of human ES cell lines, their sub-clones and in their in vitro differentiation procedures. To meet acceptance criteria, the cells are cultured with MEFs as feeder layer for at least four passages during which the following parameters are measured:
|
- Colony Morphology. The colony morphology of the undifferentiated cells is expected to remain similar to that of cells cultured with the control medium, namely round colonies with clear borders and determined by direct observation (see Figures 4&5).
- Plating Efficiency. The accepted cloning efficiency should be in the expected range which is normal for hESCs cultured with FBS and measured by counting surviving colonies (Amit et al., Dev Biol, 2000).
- Background Differentiation rates determined by morphology and determined by FACS analysis for SSEA4 for pluripotency markers.
|
Advantages |
- Only pre-screened and only those FBS batches selected that can provide the different growth factors necessary for stem cells growth promotion
- Selected FBS batches tested for consistent quality to insure batch to batch consistency.
- Promote the formation of aggregates known as embryoid bodies, important intermediates for further differentiation into neuronal or hematopoietic progenitors.
|
Mesenchymal Stem Cell Media |
|
| Product Name |
Catalogue No. |
Unit Size |
|
| Mesenchymal Stem Cell Growth Medium (Ready-to-use) |
05-300-1A |
500ml |
| Mesenchymal Stem Cell Adipogenic Differentiation Medium (Ready-to-use) |
05-301-1B |
100ml |
| Mesenchymal Stem Cell Chondrogenic Differentiation Medium (Ready-to-use) |
05-302-1B |
100ml |
| Mesenchymal Stem Cell Osteogenic Differentiation Medium (Ready-to-use) |
05-303-1B |
100ml |
|
| |
The Mesenchymal Stem Cell Growth Medium supports the expansion of multipotent human Mesenchymal Stem Cell (MSC) without inducing early senescence and differentiation as observed in standard culture media. In addition, Biological Industries offers three media to efficiently induce differentiation of MSC into adipogenic, chondrogenic, or osteogenic lineages, respectively.
|
| Recommended for: |
- Human Mesenchymal Stem Cells from Bone Marrow (hMSC-BM).Human
- Mesenchymal Stem Cells from Umbilical Cord Matrix (hMSC-UC). Human
- Mesenchymal Stem Cells from Adipose Tissue (hMSC-AT).
|
|
| Product Name |
Catalogue No. |
Unit Size |
Storage Temp. |
|
| Human Embryonic Stem Cells products |
| NutriStem™ hESC XF Xeno-Free Serum-Free medium for hESC With HSA |
05-100-1A
05-100-1B |
500ml
100ml |
-20ºC
-20ºC |
| AF NutriStem™ hESC XF Xeno-Free Serum-Free medium for hESC Without HSA |
05-102-1A
05-102-1B |
500ml
100ml |
-20ºC
-20ºC |
| Bio-Pure Human Serum Albumin (HSA Solution, 10%), Optimized for Human Embryonic Stem Cells (hESC) |
05-720-1B
05-720-1E |
100ml
50ml |
-20ºC
-20ºC |
| Mouse Embryonic Stem Cells products |
| Mouse Embryonic Stem Cells (ESC) Basal Medium, with L-Alanyl L-Glutamine |
01-171-1A
01-171-1B |
500ml
100ml |
2-8ºC
2-8ºC |
| Gelatin Solution (0.1%) |
01-944-1A
01-944-1B |
500ml
100ml |
2-8ºC
2-8ºC |
| Foetal Bovine Serum Qualified for hESC |
| Certified Foetal Bovine Serum (FBS) Qualified for Human Embryonic Stem Cells |
04-002-1A
04-002-1B |
500ml
100ml |
-20ºC
-20ºC |
| Certified Foetal Bovine Serum (FBS) Qualified for Human Embryonic Stem Cells Heat Inactivated |
04-222-1A
04-222-1B |
500ml
100ml |
-20ºC
-20ºC |
| Mesenchymal Stem Cell Media |
| Mesenchymal Stem Cell Growth Medium (Ready-to-use) |
05-300-1A |
500ml |
2-8ºC |
| Mesenchymal Stem Cell Adipogenic Differentiation Medium (Ready-to-use) |
05-301-1B |
100ml |
2-8ºC |
| Mesenchymal Stem Cell Chondrogenic Differentiation Medium (Ready-to-use) |
05-302-1B |
100ml |
2-8ºC |
| Mesenchymal Stem Cell Osteogenic Differentiation Medium (Ready-to-use) |
05-303-1B |
100ml |
2-8ºC |
| Related Solutions |
| Dulbecco's Modified Eagle Medium (DMEM): Nutrient Mixture F-12 (Ham's) (1:1) Without L-Glutamine With Sodium Bicarbonate 1.2gm/l With Hepes 15mM With Sodium Pyruvate 55mg/l |
01-170-1A
01-170-1B |
500ml
100ml |
2-8ºC
2-8ºC |
| MEM Non-Essential Amino Acids Solution, 100X Conc. |
01-340-1B |
100ml |
2-8ºC |
| Human Recombinant Insulin Solution, 3.7 mg/ml |
01-818-1H |
5ml |
2-8ºC |
| Serum-Free Cell Freezing Medium * |
05-065-1A
05-065-1C |
500ml
20ml |
2-8ºC
2-8ºC |
| L-Glutamine Solution, 29.2mg/ml in Saline, 200 mM |
03-020-1A
03-020-1B
03-020-1C |
500ml
100ml
20ml |
-20ºC
-20ºC
-20ºC |
| L-Alanyl-L-Glutamine (Stable Glutamine), 200 mM ** |
03-022-1B
03-022-1C |
100ml
20ml |
-20ºC
-20ºC |
| Sodium Pyruvate Solution, 11.0mg/ml (100 mM) |
03-042-1B |
100ml |
-20ºC |
| Crystalline Trypsin Solution (0.02%) Without Phenol Red * |
03-047-1A
03-047-1B |
500ml
100ml |
-20ºC
-20ºC |
| Soybean Trypsin Inhibitor 50X Conc., 5mg/ml * |
03-048-1C |
20ml |
-20ºC |
| Cell Dissociation Solution (non-enzymatic) * |
03-071-1B |
100ml |
2-8ºC |
| Papain Dissociation Solution * |
03-072-1B |
100ml |
-20ºC |
| Fibronectin Solution (Bovine), 1mg/ml * |
03-0901-01
03-0901-05 |
1ml
5ml |
2-8ºC
2-8ºC |
| Accutase Solution, primary human cell culture tested * |
03-073-1B |
100ml |
-20ºC |
| Transferrin, Human, Substantially Iron-Free (APO) *** |
41-951-100
41-951-500 |
100 mg
500 mg |
2-8ºC
2-8ºC |
| Transferrin, Human, Iron-Saturated (HOLO) *** |
41-952-100
41-952-500 |
100 mg
500 mg |
2-8ºC
2-8ºC |
| Insulin, Human Recombinant |
41-975-100 |
100 mg |
|
| Basic Fibroblast Growth Factor (FGF) |
30-T-218A
30-T-218B |
10μg
50μg |
|
|
| |
