User Bulletin
Viral RNA Extraction Miniprep System
For biological fluids containing RNA virus: serum, plasma, body fluids, and cell culture supernatant.
Downstream Application
- Northern, dot and slot blotting
- RT-PCR / Quantitative real-time PCR
- Poly A+ RNA selection
- cDNA Synthesis/ Primer extension
- Array analysis
- In vitro translation
Product Contents
|
Cat No
|
RNV52-904LT
|
RNV52-906LT
|
|
AuPrePT Preps
|
50
|
250
|
|
AuPrePT RXV Buffer
|
35ml
|
190ml
|
|
AuPrePT WS Buffer
|
15ml
|
45ml x 2
|
|
AuPrePT RNA Carrier
|
1
|
1
|
|
AuPrePT Proteinase K
|
10mg
|
3x10mg
|
|
AuPrePT RNase-free ddH2O
|
1.5ml x 2
|
15ml
|
|
AuPrePT RNA Mini Column
|
50
|
250
|
|
AuPrePT Collection Tube
|
50
|
250
|
|
AuPrePT Protocol
|
1
|
1
|
|
All buffers need to be mixed well before use.
|
Shipping & Storage
AuPrePT Viral RNA Extraction System is shipped at ambient temperature and stored for up to 6 months.
If precipitation forms by freezing temperature on any buffer, warm up at 37°C to redissolve.
Protocol
- Please read the following notes before starting the procedure.
- WARNING, strong acids and oxidants (for instance, bleach) should not be used together with RXV buffer (because this kind of reaction
would produce toxic cyanide)!!!
Important Notes
<Note>:
Preheat RNase-free ddH2O to 80°C.
-
Add 1 ml sterile ddH2O to each tube to reconstitute the provided Proteinase K by vortexing. Store the solution at 4°C.
- for RNV52-904LT-
Add 60 ml of ethanol (98-100%) to the WS Buffer bottle when first open the bottle.
- for RNV52-906LT-
Add 180 ml of ethanol (98-100%) to the WS Buffer bottle when first open the bottle.
Special design Lid -
Designed to prevent contamination during the procedure.
Tips for
Lid
-
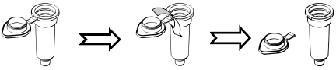
Twist the arm of the cap and pull the cap to break the Lid.
1.
Add RNA carrier to RXV Buffer.
Add 1 ml RXV Buffer to the RNA carrier tube, vortex to dissolve and transfer to the RXV Buffer bottle, store at 4°C.
2.
Pipet 150 µl sample (serum, plasma, body fluids, and cell culture supernatant) into a 1.5 ml tube.
3.
Add 570 µl of carrier added RXV Buffer to the sample, mix by vortexing.
Through mixing is required for sample lysis. If the sample volume is larger than 150 µl, increase the amount of RXV buffer proportionally.
4.
Add 10 µl Proteinase K to the sample and incubate at Room Temperature for 10 minutes
5.
Add 570 µl of ethanol (98-100%) to the sample, and mix by vortexing.
If the starting sample is larger than 150 µl, increase the amount of ethanol proportionally.
6.
Place a RNA Column in a 2 ml Collection Tube, apply 650 µl of the ethanol added sample from step 5 to the RNA Column, close the cap, centrifuge at
6,000 x g (8,000 rpm) for 1 minute, and discard the filtrate.
If the solution remains above the membrane, centrifuge again at 13,000 rpm.
7.
Repeat step 6 for rest of the sample.
8.
Wash the column twice with 500 µl of ethanol added WS Buffer by centrifuging at full speed (13,000 rpm or 10,000 x g) for 1 minute, and discard the
filtrate.
Add 60 ml (for RNV52-904LT) or 180ml (for RNV52-906LT) of ethanol (98- 100%) to the WS Buffer bottle when first open the bottle.
9.
Centrifuge at full speed for 5 minutes to remove traces of WS Buffer.
Residual ethanol may cause the low A260/A230 and inhibit reverse transcriptase activity.
10.
Transfer the column to a RNase-free 1.5 ml tube (not provided), add 50 µl of preheated (80°C) RNase-free ddH2O, and centrifuge at full speed
for 1 minute to elute RNA.
11.
Store RNA at -70°C.
| 